|
| William Robert Grove (1811-1896)
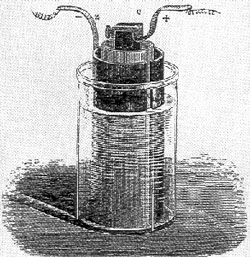
invented two cells of special significance. His first cell consisted
of zinc in dilute sulfuric acid and platinum in concentrated nitric
acid, separated by a porous pot (Grove Cell). This cell had nearly
double the voltage of the first Daniell cell. His second cell, a
“gas voltaic battery” was the forerunner of modern fuel
cells. Grove was born in Swansea, Wales in 1811. He was a professor of physics in the London Institution from 1841 to 1846. He also served as a barrister and a judge of the Court of Common Pleas and of the High Court of Justice. Grove was the first to show that electrolysis, with a high-tension current, can take place through thin glass. The first experimental proof of dissociation was given by Grove, who showed that steam in contact with a strongly heated platinum wire decomposed into hydrogen and oxygen. His nitric acid cell was
the favorite battery of the early American telegraph (1840-1860),
because it offered strong current output. By the time of the American
Civil war, Grove’s battery was replaced by the Daniell battery.
As the telegraph traffic increased, it was found that the Grove
cell discharged poisonous nitric dioxide gas. Large telegraph offices
were filled with gas from rows of hissing Grove batteries. As telegraphs
became more complex, the need for constant voltage became critical
and the Grove device was necessarily limited (as the cell discharged,
nitric acid was depleted and voltage and reduced). |