|
| Johann Wilhelm Ritter (1776-1810) discovered
the ultraviolet end of the spectrum, made the first dry cell battery
in 1802 and a storage battery in 1803. His most important contribution
to electrochemistry came in 1798. Ritter was the first to establish
an explicit connection between galvanism and chemical reactivity.
He correlated the electrical effects produced by various metal couples
on the muscle with differences in the metals’s ease of oxidation.
His suggestion that current was due to a chemical interaction between
the metals was the first electrochemical explanation of this phenomenon.
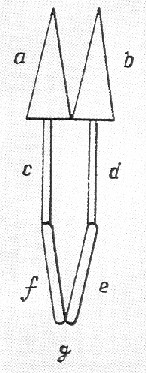
The apparatus shown is Ritter’s device for detecting direction
of current flow between metals and conductive frog thighs. Ritter was born in Samitz, Silesia (now Poland) ane began his career as an apothecary. He went to the University of Jena in 1796 to pursue his interests in science. It was at Jena that he experimented with silver chloride. Although it was known that AgCl decomposed in light, Ritter found that this process was most efficient in the presence of “invisible” radiation, beyond the violet end of the spectrum. This radiation became known as ultraviolet radiation. Ritter was fascinated by experiments in electrical
excitation of muscle and sensory organs as well as the electrophysiology
of plants. Much of his success in such studies was due to use of
his own body, even at very high voltages. Such work may have exacted
a high, personal toll from Ritter. He became very ill. While embroiled
in several controversies as a member of the Bavarian Academy of
Sciences (Munich), he died at the young age of 33. |